ISAC: Molecular Allergy Panel
A brand new method that takes allergy diagnosis to a far superior level.
In Molecular Allergology, sensitization to specific allergen components is measured, which gives the patient’s specific IgE profile in detail. In this way, symptoms due to cross-reaction are explained and help to assess risks in the management of the patient.
ISAC on the other hand is enables the simultaneous detection of 112 allergen components from 48 different main allergen sources. While shedding light on the true sensitization profile of susceptible patients, under favour of cross-reacting proteins,
ISAC can provide information on hundreds of allergenic components in addition to the 48 main allergen sources from which the proteins are derived.

ISAC: Molecular Allergy Panel – 14.05.2018 – SCIENTIFIC BULLETINS – Biruni Laboratory – 0850 241 77 88
Allergy is an overreaction of the body to a normally harmless substance and means “different reaction” in ancient Greek. Allergens are usually protein or glycoprotein, rarely polysaccharide macromolecules, triggering the IgE antibody response in humans.
In recent years; Due to the increasing frequency of allergic diseases, the diagnostic approach gains great importance. In the diagnosis of allergic diseases, as in all diseases, the first step is to take a good history, in which the patient’s symptoms are questioned in detail, and to perform a systemic examination, especially in search of clinical signs of allergic diseases. It is extremely important to request appropriate laboratory tests and their clinical interpretation after the history and physical examination.
Allergies are a huge health and socioeconomic burden worldwide, especially in industrialized countries. More than 40% of the population in Europe still suffers from at least one allergy. Approximately 70% of these allergic patients are polysensitized. Children are often affected by atopic dermatitis, allergic rhinitis and allergic asthma. High-accuracy in vitro diagnoses that complement conventional diagnoses; essential for optimal patient management and effective treatment. Multiplex systems streamline treatment procedures by presenting a comprehensive and detailed patient profile in a single test.
Specific IgE antibodies appear in human serum and plasma as a result of sensitization to a specific allergen. Measurement of circulating IgE antibodies provides an objective assessment of sensitivity to an allergen. In general, low IgE antibody levels indicate a low probability of clinical disease, while high antibody levels to the allergen correlate with clinical disease.
Clinical Value of Quantitative Test
- An increase in IgE antibodies, sensitization can be detected at an early stage before clinical symptoms occur.
- It helps in understanding the progression of allergic disease.
- It helps explain the burden of the allergen.
- It allows the determination of the appropriate treatment for the management of the disease.
ImmunoCAP Major Allergens and ImmunoCAP Components
The diagnosis of allergy is based on the patient’s detailed case history, clinical observations, and the results of the Specific IgE test. Using ImmunoCAP Allergens or ImmunoCAP Allergen Components to detect the presence of IgE antibodies provides the ability to detect major allergens or allergen subcomponents to aid in reliable diagnosis of suspected allergy patient.
Knowing the specific IgE antibody levels provides guidance below on:
- Determining the appropriate individual treatment method for each patient
- Reducing exposure to the target allergen
- Follow-up of the development of tolerance (food allergy, specific immunotherapy)
- Facilitate optimized individual medical treatment plans (time and dose)
Clinical Value of ImmunoCAP Major Allergens
The results from the ImmunoCAP allergy test are used to detect the specific allergen causing the allergic reaction and to eliminate the allergens that are negative. The results may also be helpful in monitoring specific IgE levels of antibodies over time. An increase in IgE antibodies can be detected at early diagnosis, indicating sensitization before clinical symptoms develop and helping to identify patients at risk for the below on:
- Allergy state of affairs – Transform of skin symptoms to respiratory symptoms
- Transform of mild symptoms to severe symptoms
- Chronicity – Transform of recurring symptoms to persistent symptoms
Clinical Value of ImmunoCAP Allergen Components
Molecular Allergology is the state-of-the-art approach to allergy diagnosis. The single allergen components identified here are used for the detection of specific IgEs based on molecular protein, rather than the traditionally used allergen extracts (Figure 1).
Individual allergen components are highly purified proteins isolated directly from the allergen source or produced recombinantly. The sensitivities developed against these components are measured with separate individual tests to determine which component the patient is sensitive to at the sharp molecular level. This provides a higher level of standardization and differential diagnosis than tests based on allergen extracts. Molecular Allergology systems are a powerful diagnostic tool that facilitates risk assessment and treatment decisions, as they can identify the sensitive trigger of allergy.
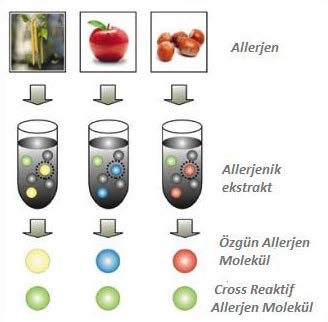
Figure 1. Specific allergen components from a basic allergen source.
What can allergen components tell us?
Allergen components are proteins that have structural similarity and are grouped under different protein families. The reason for the sensitivity developed against these components, which are found in different amounts in the sources and have different stability is due to their common group characteristics. Depending on the properties of these proteins, the sensitization developed by the patient produces different results. Some allergen components are specific and some are cross-reactive.
What Other Contribution Does Molecular Allergology Provide?
With Molecular Allergology, specific allergen components can be produced from a basic allergen source for advanced diagnosis. Sensitization to these components is measured individually in a separate test and at a precise molecular level it is determined which component the patient is sensitive to. This information provides the basis for the highly sensitive diagnosis of allergy. In Molecular Allergology, extract-based tests are used together with component-specific analyses.
While the extract gives the answer to which allergen source the patient is sensitizing to, the allergen components provide vital information about risk, specificity and cross-reaction.
1) Determines the risk of clinical reaction
Molecular Allergology draws conclusions on the risk associated with sensitization. Sensitization to stable allergen components can elicit local as well as systemic reactions, while sensitization to unstable components is mainly associated with local reactions.
Şekil 2. Risk levels relevant with common allergen proteins
Specific components – provide unique clues to reveal sources of allergies.
Each allergen source typically contains both specific and cross-reactive allergen components. Specific allergen components are almost uniquely related to their source and are only present in small amounts in a limited number of closely related species. Each allergen source may contain one or more specific allergen components. Sensitization to any of these indicates a true sensitization in the person; this means that the relevant allergen source is the primary cause of clinical symptoms.
2) Explains symptoms due to cross-reaction
Symptoms elicited by cross-reacting antibodies can be distinguished from those caused by true sensitization, which is important for patient management and appropriate avoidance advice. Where only cross-reaction sensitization is detected, it is necessary to find the primary sensitizer.
Identification of cross-reactive components
It is important to identify the exact trigger of the allergy before starting treatment. However, patients; Cross-reactivity of allergens is common in skin tests and clinical tests such as extract-based antibody tests. Analysis of allergen sources and components of each of the most important pan-allergens allows rapid and accurate identification of the exact triggers of allergy symptoms.
The cross-reaction may be exemplified by birch pollen-related food allergy, a syndrome that affects many birch pollen allergy sufferers. The molecular reason underlying the cross-reaction is that patients with birch pollen allergy have specific IgE antibodies specific to the Bet v 1 component. Bet v 1 has structural similarity to related proteins in many foods, such as soy and peanuts. In this way, the patient’s IgE antibodies to Bet v 1 birch cross-react with these related proteins in soy or peanut.
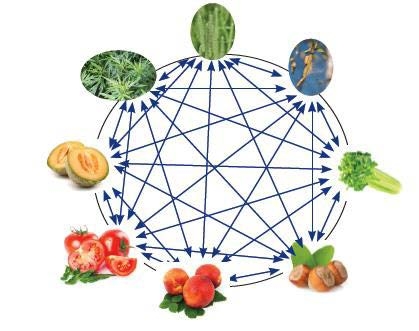
Figure 3: PR-10, Protein- is sensitive to heat and digestion. Cooked foods are often tolerated. It is mostly associated with local symptoms such as OAS. It is related with allergic reactions to pollen, fruits and vegetables.
3) Selection of Appropriate Therapy – Contributes to identifying the right patients for Specific Immunotherapy (SIT).
Determination of sensitization to specific allergen components is necessary for successful Specific Immunotherapy. In the light of these findings, the outcome of the treatment to be applied in patients who develop a true sensitization against the component extracted from the relevant allergen source will be positive. SITs are most likely to be successful when the patient is primarily sensitized to the main components of the allergen extracts. Only molecular allergy diagnoses can offer such in-depth information. The most appropriate treatment can then be selected and patients are relieved of the stress of avoiding unnecessary allergens or ineffective SITs.
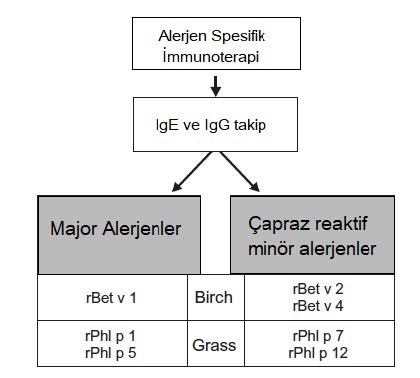
Figure 4 : Recombinant allergen-based diagnostic tests and specific immunotherapy specific to birch and meadow grass allergen
Protein Stability and Amount
Food allergen components show different stability to heating and digestion, and their content in the allergen source may vary. Both stability and quantity are reflected by the protein family to which the component belongs. Therefore, it is possible to assess the risk associated with sensitizations by knowing the patient’s sensitization profile and to which family the identified components belong.
 |
Figure 5 : Immunoglobulin E (IgE) levels of allergen molecules based on structural similarity in an allergen family. a. 2S albumıns (fındık, bakliyat ve tohumlarda stabil depolama proteinleri) arasında değişken, sınırlı çapraz reaktivite. b. Bet v 1-PR-10 variable cross-reactivity among homologous food allergens. c. High cross-reactivity (in pollen, latex and foods) due to the strongly preserved and similar structure of the Profilins |
For example, in egg allergy, Gal d1 (ovomucoid) is the main allergen and is used as an indicator of allergic reaction severity. Sensitizations to the heat-sensitive components Gal d2 (ovalbumin), Gal d3 (conalbumin), and Gal d4 (lysozyme) are associated with symptoms that occur only with consumption of raw or lightly cooked eggs. Ovalbumin is used in vaccines and lysozyme is used as a preservative, so patients sensitive to these ingredients may have a reaction to drugs or food products containing the relevant ingredient. Similarly, the reaction to Bos d8 (casein) indicates a strong allergy to milk and dairy products. Casein is often used as an additive, so casein sensitivities can cause sensitivities to many different foods, such as chocolate or potato chips. Bos d (lactoferrin), Bosd4, Bos d5 and Bos d6 components are heat sensitive and sensitivities to these components are mainly associated with reactions to fresh milk. Antibodies to Bos d6 (bovine serum albumin) can also cause a reaction to beef.
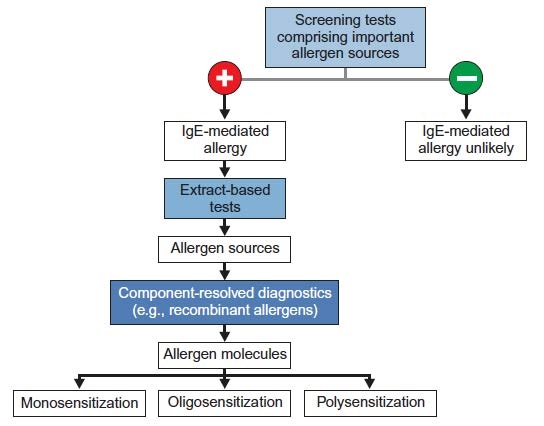
Figure 6 : Serological tests for the diagnosis of allergies
ImmunoCAP ISAC (Immuno Solid-phaseAllergenChip)
 |
Detection of IgE antibodies in serum plays an important role in the diagnosis of type I allergies. The currently used extract-based method of specific IgE antibodies, ImmunoCAP-FEIA, is the gold standard for allergy diagnosis. However, while this test only allows the simultaneous determination of a limited number of allergens, Immuno CAP ISAC enables the simultaneous detection of 112 allergen components from 48 different main allergen sources. This system is array-based, biochip technology and is the most advanced in vitro diagnostic test for the measurement of specific IgE antibodies for allergen components. The ISAC test facilitates the identification of a comprehensive and specific IgE antibody profile. ImmunoCAP ISAC is a semi-quantitative test and results report specific IgE antibody levels in ISAC Standard Units (ISU) within the 0.3 – 100 ISU-E measuring range. |
ISAC Advantages:
Simultaneous analysis of 112 allergenic proteins allows us to fully and quickly identify the allergens causing the patient’s clinical condition from a single blood sample. The very wide range of allergenic protein studies makes it possible to highlight unexpected sensitizations and/or rule out others. As a result, the analysis allows to obtain an individual sensitization profile with improvement in diagnosis, leading to an individualized treatment. While all this helps improve the patient’s quality of life, the economic cost of analysis is much less than analyzing individual specific IgEs. Most allergic patients test positive to numerous allergens, and the true cause of symptoms may not be determined due to an uncertain medical history regarding the role of different allergens and reactions.
ImmunoCAP ISAC in the diagnosis and treatment of these patients;
- It sheds light on the true sensitization profile of susceptible patients.
- It presents the potential risk for severe food-related reactions.
- It determines the IgE antibody profile in patients with inadequate response to treatment.
Under favour of cross-reacting proteins, ImmunoCAP ISAC can provide information on hundreds of allergen components in addition to the 48 main allergen sources from which the proteins are derived. ImmunoCAP ISAC can reveal unexpected sensitivities or help rule out allergy by providing IgE results for a wide array of allergens. As a result, effective and optimized treatment principles for patients can be initiated earlier and the quality of life is increased while ensuring patient health.
Cross-reacting allergen components are more widely distributed and can be shared among a wide range of allergen sources. Due to their high structural similarity, they can cause cross-reactivity of the IgE antibody.
Components from different protein families produce symptoms of varying severity. Thus, molecular profiling can determine whether a patient has a low or high risk of serious systemic reactions such as anaphylactic shock. Patients at risk of life-threatening reactions can then be advised on allergen avoidance and appropriate measures to be taken in an emergency. For example, if a patient is sensitive to allergens in the profilins family, milder symptoms can be expected overall. Patients who are sensitive to allergens belonging to the family of storage proteins have a high risk of life-threatening systemic reactions. In addition, differences in the heat resistance of protein families play an important role in food allergies.
The prevalence of oral allergy syndrome (OAS) is increasing. It is currently reported to affect approximately 2% of the population in primary care practices in the UK. Although it can often be diagnosed by clinical history, the need for confirmatory testing is increasing for patients with typical oral symptoms after eating raw fruits or vegetables, or sometimes raw nuts. The overlap of symptoms between patients with oral allergy syndrome and patients with nut allergy is an area that often requires more precise definition using available tests to inform patient management and stratify risk. Testing multiple recombinant proteins with ISAC is particularly useful in elucidating patients’ reaction to PR-10 and/or profilin proteins (combined low risk of clinical reaction only) or lipid transfer protein or peanut storage proteins (higher risk of severe reaction).
ISAC Indications:
- Improving diagnosis in polysensitized patients.
- To prevent diagnostic errors, especially in patients who do not have a clear correlation between the positive results of conventional allergy tests and symptoms.
- Preventing therapeutic errors that may occur in terms of Allergen Specific Immunotherapy.
- To evaluate cases with an inconsistent clinical history of allergy and/or inadequate response to treatment.
- Evaluation of patients with a history of idiopathic anaphylaxis detecting unexpected sensitivities. ISAC ALLERGEN PROTEIN FAMILIES
ImmunoCAP ISAC provides a large amount of allergen-specific IgE antibody information in a single step. Thanks to cross-reacting proteins, ImmunoCAP ISAC can provide information on hundreds of allergen sources in addition to the 48 sources from which the proteins are derived.
Please Click to overview the ISAC Immunocap Cross-Reaction Map
|
Storage proteins |
|
|
Profilin (1) |
|
|
PR-10 protein, Bet v 1 homologue(1) |
|
|
Polcalcin (Calcium-binding proteins) (2) |
|
|
LTP (non-specific Lipid Transfer Proteins, nsLTP)(1) |
|
|
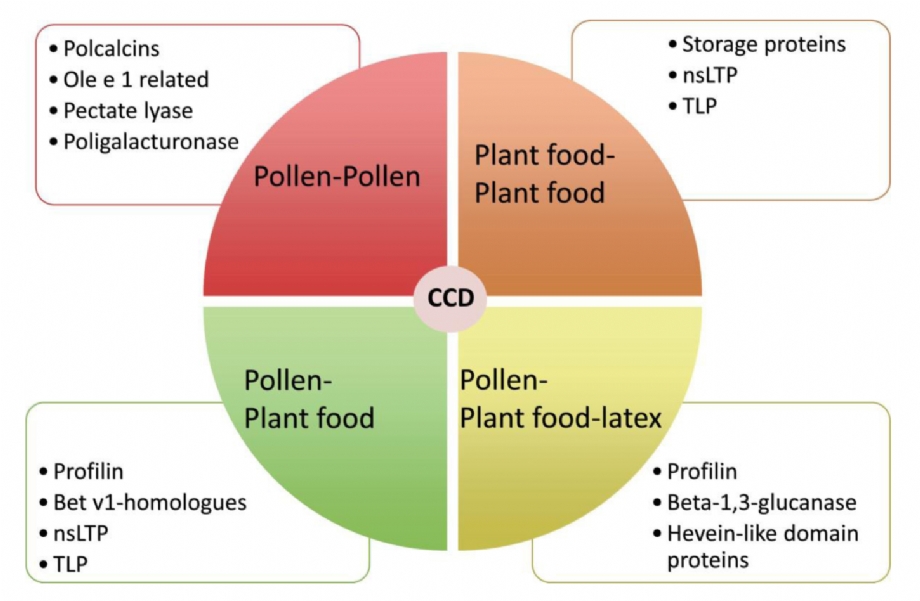
CCD (2) |
|
|
Lipocalin (3) |
|
|
Parvalbumin (3) |
|
|
Serum albumin (3,4) |
|
ISAC IMPORTANT ALLERGEN COMPONENTS
|
Gal d 1, Ovomucoid (egg white / glair) (3) |
|
|
Tropomyosin (3) |
|
|
Ara h 1, 2, 3, 6, 8 and 9 (peanut) (3) |
|
|
Gly m 4, 5 and 6 (soy) (3) |
|
|
Alt a 1 (Alternaria) (3) |
|
|
Tri a 19, Omega-5 gliadin (wheat) (5,6,7) |
|